The starting point of this article about artificial photosynthesis is an earlier article in which we looked at the environmental impacts of solar energy and its contribution to greenhouse gas (GHG) emissions.
Even though – with 46g/ kWh – the GHGe (greenhouse gas equivalent) of solar PV is significantly lower than the GHGe of oil (893g/ kWh) and coal (up to 994g/ kWh) and thus solar PV is certainly one of the major ways to think about energy in our near and distant future, still PV contributes to GHG.
How can we further reduce this? And how can we use the sunlight even more efficiently, more flexibly?
The high potential answer is very close to us – we inhale and exhale it actually day per day.
Scientists are working on the next solar energy revolution, tapping into biochemistry by mimicking the wondersome process our blue planet: photosynthesis.
What is Photosynthesis?
Photosynthesis refers to the most important biochemical process on our planet that makes life actually possible.
During this complex process – which also diverges from one specias to the other – specific proteins in photoautotrophs such as plants and algae harvest sunlight energy in form of photons and transform it into a form of chemical energy which is called adenosine triphosphate (ATP), in complex chemical terms: C10H16N5O13P3.
The ATP is then used for the synthesis of sugars (carbohydrates) from water (H2O) and carbon dioxide (CO2), releasing oxygen (O2).
Artificial photosynthesis – technology research background
While many forms of life on earth depend on photosynthesis to exist, chemists and energy economists are exploring on the vast energy potential geared to the post-photosynthetic release of hydrogen and oxygen.
Scientists all over the globe are currently working on solutions to utilize, improve and integrate photosynthesis by creating devices that imitate this process, thus achieving artificial photosynthesis.
Technology approach: splitting water
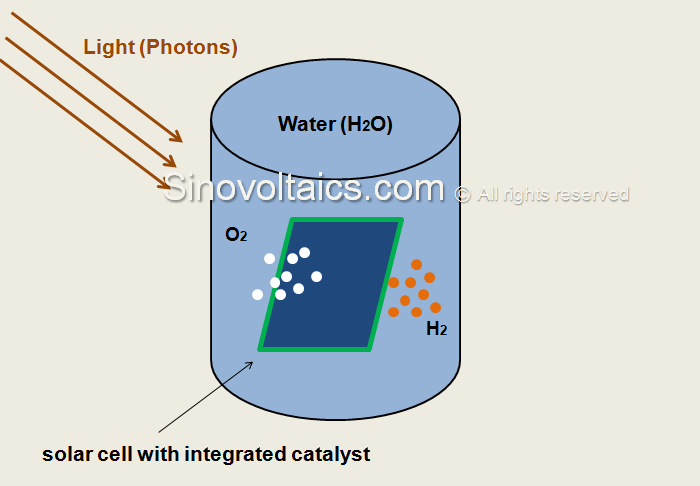
One prominent example is an artificial leaf invented by famous chemist Daniel Nocera from Harvard University which imitates photosynthesis.
Nocera’s artificial photosynthesis leaf is simply a silicon wafer which features a cobalt-based layer on one side and a nickel-molybdenum-zinc alloy on the other side. An earlier model featured platinum as catalyst which is however more expensive.
The silicon junction then utilizes incoming photons to energize a process during water surrounding the artificial leaf is split into hydrogen which is released from the nickel-molybdenum-zinc alloy side, and oxygen which is released from the cobalt side.
In this process, usable gases are produced for fuel cells which can be burned in power plants and thus turned into electricity.
Technology approach: chemical reduction of CO2
Another artificial photosynthesis approach has been undertaken by engineers at the University of Illinois at Chicago (UIC). They have been researching on an artificial solar leaf that converts carbon dioxide into combustible fuel.
There are no complicated intermediate processing stages. The leaves absorb carbon dioxide and release syngas that can be used as a fuel, and oxygen that purifies the atmosphere at the same time.
A major hurdle has been chemical reduction of CO2 (removing an oxygen from its molecule) for which very costly catalysts like silver were previously used.
The engineers at UIC however managed to find a much better catalyst in the form of a transition metal compound which is claimed to be twenty times cheaper, yet a thousand times faster.
The process is certainly green whether the ‘leaves’ are green or not in color.
What is Syngas?
Syngas – which is an abbreviation for synthesis gas – is a mixture of hydrogen, carbon monoxide, and traces of carbon dioxide.
The name syngas came from the fact that this mixture is an intermediate stage in the production of synthetic natural gas, and other products like ammonia, and methanol (another alternate fuel).
It can also be burnt directly, or converted to diesel or other forms of fuel. A key question is: can we turn atmospheric carbon dioxide, a major greenhouse gas, into fuel at a cost comparable with that of gasoline?
This could make fossil fuels irrelevant, and thus contribute to energy security. It would also help check, and even reverse global warming.
Pros, cons and potential applications of artifical photosynthesis
The possibilities presented by those artificial photosynthesis technologies are enormous, and can potentially revolutionize not only the way we generate electricity, but also the ways we travel.
Imagine green automobiles that produce their own fuels. Isn’t that wishful thinking? Having automobiles which generate fuel for them while on the go!
Do you think a solar forest of ‘trees’ with such solar leafs could form a health resort, while supplying cheap energy?
On a small scale it could even be used at home. A beautiful artificial tree can purify the inside air, enrich it with oxygen, and produce fuel for the burner.
The approach of the engineers at UIC could be particularly useful in space travel. The carbon dioxide released by breathing of astronauts could be converted back to oxygen while gaining fuel.
We know forests breathe out oxygen. But now there is prospect of developing artificial solar forests which can breathe out not only oxygen, but also syngas, a fuel gas that has many end uses.
And a major benefit is the effect on global warming. Not only is it free of GHG addition, it actually reverses GHG concentration by absorbing CO2 from the atmosphere.
However, to make these technologies relevant and potentially replace fossil fuels, they must be scaled up to commercial level. Furthermore, the current high costs must be further reduced so to make it competitive to biofuel.
Nevertheless, the invention could have far reaching effects on our life style, economy, and policies on environment. That is why the research is being supported by the U.S. Department of Energy and the National Science Foundation.
The design has been tested using artificial solar light. But the designers are so confident of what they have achieved that a provisional patent application has already been made.
Sources and further reading:
1) Lynn Savage: Artificial Photosynthesis: Saving Solar Energy for a Rainy Day. Optics & Photonics News, February 2013. URL: http://www.osa-opn.org/home/articles/volume_24/february_2013/features/artificial_photosynthesis_
saving_solar_energy_for/
2) Mohammad Asadi et al.: Nanostructured transition metal dichalcogenideelectrocatalysts for CO2 reduction in ionic liquid. Science, July 2016 DOI: 10.1126/science.aaf4767