In the previous articles, we have already discussed a variety of solar energy storage technologies, including conventional and non-conventional battery cell technologies.
After we previously covered thermal batteries, we continue this time with another special, non-conventional battery technology type: the flow battery.
We will explain the key features of flow batteries, how they work, its pros and cons as well as introduce the different subtypes of flow batteries.
What is a flow battery and how does it work?
Originating in Germany, flow batteries, also called liquid flow batteries, can be categorized as a subtype of regenerative fuel cells, yet they also feature key electrochemical properties and functional principles of conventional battery cells: reversible electrochemical reactions.
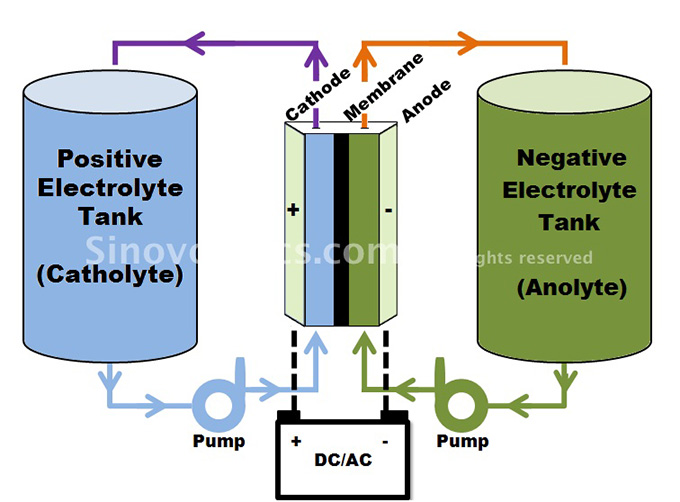
The structural design of a flow battery is however different. Unlike conventional battery cell technologies, a flow battery stores the energy as electrolytes and not as electrodes.
The oppositely charged electrolytes are made of electroactive solvents, which are elements in a solution that can react with the electrodes. These are stored outside in two separate tanks instead of inside the core.
One tank stores the positive electrolyte (catholyte) and the other one stores the negative electrolyte (anolyte).
Both tanks are connected via separate pipes and pumps with the core which itself consists of (usual graphite) cathode and anode plates and a membrane between them.
The charge and discharge process of a flow battery basically involves the pumping of the electrolytes through the core, each flowing in separate circuits past each electrode side.
During discharge, the electrolytes are pumped from the tanks into the core, electrochemically reacting with the cathode and anode respectively and ion exchange takes place through the membrane. In this flow process, chemical energy is converted into electric energy. When charging, the electrolytes are pumped out of the core back into the tank.
The working principle of a flow battery is similar to that of a combustion engine which uses fuel stored in storage tanks, however with the distinct feature of re-using the “fuel.”
Different from conventional battery cells, the whole setup of flow batteries requires extra components, such as the aforementioned electrolyte tanks, pipes, pumps, sensors and related control units. Flow batteries are therefore not only more complicated and costly but also not suited for small-scale applications.
Flow battery: types and technologies
There are different types of flow batteries. The main types are reduction-oxidation (redox) flow batteries, membraneless flow batteries, organic flow batteries, and hybrid flow batteries. Below we explain in more detail the common main types:
Redox flow battery
The most common flow battery type is the redox flow battery, or also called: true redox flow battery. As already explained, the energy is stored as positive and negative electrolytes in separate tanks outside of the core. The electrodes of the core are separated by a membrane and each electrolyte flows within the positive and negative circuits respectively.
During the discharge, the flow triggers an electrochemical reaction of the electrolyte with the electrodes. The oxidation reaction on the anode releases electrons that move in the circuit and move through the thin, porous membrane to react with the cathode (reduction reaction).
On the contrary, when charging, oxidation reactions on the cathode and reduction reactions on the anode take place. The voltage and current of a redox flow cell are basically geared to the chemicals and their properties.
The commercially most common redox flow battery type is the Vanadium-redox battery which uses Acid Sulfur with Vanadium salt as an electrolyte. Vanadium is mined for example in China and Russia and is popular in the steel industry as a strengthening material because it is less erosive.
Organic flow battery
Organic flow battery cells employ the same design and functional principle as redox flow batteries, however, the difference lies in the material structure of this flow battery type.
Different from other flow battery types, organic flow battery cells employ metal-free, organic molecules that are abundant in nature and therefore cheap. These molecules are called quinones and technically resemble the molecules that store energy in plants.
With ongoing research and increasing interest in this flow battery technology, scientists from Harvard University have in recent years come up with such an organic flow battery using carbon-based molecules as electrolytes and which are capable of storing large amounts of energy and costing much less than all other flow battery types. (Harvard News)
Membraneless flow battery
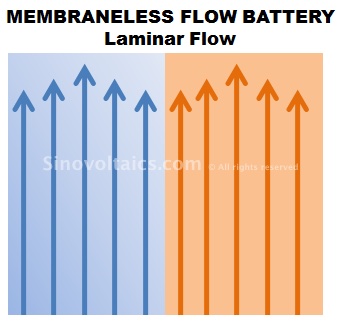
Membraneless flow cells, as the name indicates, are flow battery cells without using the otherwise sensitive and costly membrane of redox flow cells.
Quite similar in design and function to a redox flow battery cell, the electrolytes of membraneless flow cells are pumped through positive and negative circuits and trigge electrochemical reactions, however, there is no membrane.
How is this possible? Membraneless flow cells build on the principle of streamline (or: laminar) flow. This principle basically refers to the parallel stream of two liquids without mixing. This is achieved by maintaining a rather low flow speed of the liquids.
Hybrid flow battery
Hybrid flow batteries, as the name suggests, are a hybrid, ‘non-true’ form of (redox) flow batteries. With hybrid flow batteries, the electrolytes are stored in external tanks and then during charge and discharge pumped through the core. Yet similar to conventional secondary battery technologies, one electrolyte is electroplated on the electrodes in the cell core.
Hybrid flow cell types most prominently include Zinc-Bromine and Zinc-Cerium. With the redox reactions taking place at the electrodes, during the charging process, the metallic zinc from the anolyte is plated on the anode thus storing the energy there.
For this reason, and unlike true redox flow batteries, energy storing and power are not fully separated. Moreover, it limits the scalability feature since a large energy storage capacity requires a larger battery cell core.
Even though the elements used are cheaper than the Vanadium in standard redox flow cells, the problem with Zinc-based technologies is however the built-up of zinc residues at the very costly membrane, gradually leading to corrosion and thus reducing efficiency over time.
Other flow battery types
With ongoing research, there are more and more flow battery types. Such as for example the semi-solid flow battery, the nano-network flow battery, and the metal hydride flow battery.
Semi-solid batteries are a bit different in design as the positive and negative electrodes are suspended in a liquid. These molassic suspensions consisting of partly solid particles are stored in a positive and negative charge tank. The charges are then pumped via separate channels through a reaction chamber which, similar to the standard redox flow cell design, is separated by a porous membrane.
In the relatively new nano-network flow batteries, nanoparticles are used to make electricity flow through the liquid carrier instead of employing the movement of charges during the reactions of particles with the electrode plates.
With metal hydride flow batteries, water is split and hydrogen ions are produced that are then combined with the metal particles of the electrodes. Similar to hybrid flow batteries, during charge energy is stored in form of a solid metal hydride. During discharge, the oxidation of the hydrogen generates water and electricity with which connected loads are powered.
Common advantages of flow batteries
The main advantage of flow batteries is their scalability. The energy density is basically determined by the electrolyte volume – the size of the storage tanks – as well as the surface area of the electrodes within the core. By using larger tanks that can store more electrolytes, the capacity of a flow battery can thus be easily increased.
The design and modular scalability of flow batteries also allow for quick discharges and charges, such as by replacing the electrolytes.
Flow batteries can stay idle for a very long time without losing charge. Other key features of flow batteries are their basically decades-long (or even claimed unlimited) lifetime as the electrolyte can basically be re-used over and over again. This also saves costs in the long run.
Flow batteries also stand out for their fast response times, quite low harmful emissions, low maintenance requirements, and tolerance for overcharging and over-discharging.
Generally, because of their structural and functional design, flow batteries are not suited for quick power generation but rather suited for the storage of bulk energy and are therefore an interesting option as grid-energy, and specifically solar energy storage technology.
Disadvantages of flow batteries
A flow battery certainly has many advantages, however, there are (still) quite some disadvantages with flow batteries. They have on average lower power density and are more complex.
That complexity is related to the various components required and the functional design, making the battery comparably larger. Because of their bigger sizing, weigh comparably more and are not an interesting alternative for small-scale consumer electronics.
Being mechanically activated by pumps, they are therefore rather best suited for large size applications from several kW even to MW. Japan has since the 1990s installed several MW-large flow battery systems.
Moreover, the components and chemicals used with most flow battery technologies are still comparably expensive, keeping their costs at a similar level to Lithium-ion batteries, especially when looking at small-scale applications.
This is unless they are used for large-scale grid-storage applications which make them quite economical.
Nevertheless, organic flow batteries are a further step into the direction of decreasing those costs.
Outlook
There is increasingly more material research going on with flow batteries, which particularly involves the testing and of new chemical species used in the systems. Companies also work on simplification and better modular handling of flow batteries: newer systems come in the size of small boxes or large containers and can be easily stacked.
Even though for the time being flow batteries will have a difficult time to be considered for small home consumer electronic devices, their prospects as large buffer storage technology for fluctuating energy production, such as solar energy and other renewables, look very promising.
TO OUR READERS:
Do you have experience with flow batteries and if yes, which type? If you would like to share your experiences with us, please feel free to comment or mail us!