Damaging Lithium Ion Batteries
Would you like to learn about how lithium-ion batteries may get damaged? How to avoid such circumstances?
This matter has become an important one, since lithium ion batteries are a regular and integral part of technology in daily use. Lithium ion batteries can burst or catch fire, posing a possibility of damage to life and property. So, how can we prevent damage to these lithium ion batteries? One simple answer is that we should not to misuse them.
What comprises misuse of lithium ion batteries? There are three possible ways batteries can be misused:
1. Electrical Misuse
Electrical misuse is the most common way to damage your lithium batteries. Both over charge and over discharge are harmful.
Overcharge
Just as overfeeding you child is bad, overcharge can be bad for the health of lithium batteries. Overcharging, say above a voltage of about 4.2 volt will cause excessive current. The resulting over-current leads to a phenomenon called plating. The high rate of lithium ions travelling to the carbon anode cannot be accommodated by the anode intercalation layers and lithium metal tends to deposit as a lithium plating on the anode surface. This has two harmful effects:
- The strength of the current carrying force, the free lithium ions, decreases. An irreversible capacity loss results.
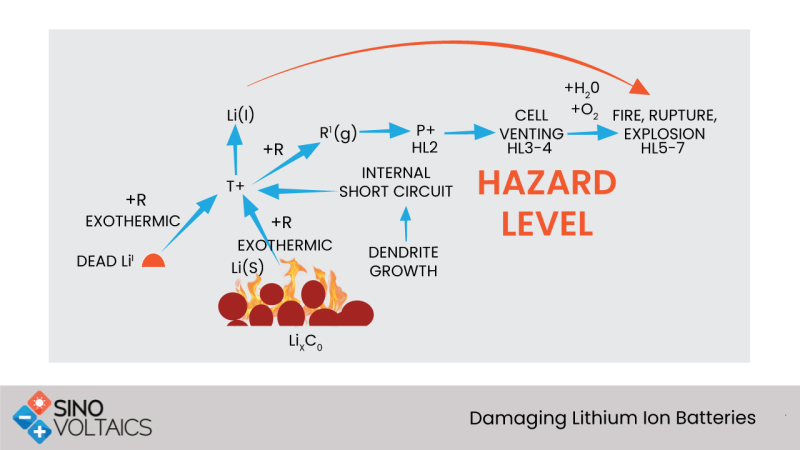
- The plating depth is uneven and tends accumulate at random points developing dendrites. These dendrites could lead to a short circuit across the battery.
Over Discharge /Undervoltage Operation
- Similarly, operating at low voltage levels also damages the battery. Extended operation below about 2V, causes a gradual breakdown of the electrode structures.
2. Under-voltage / Over-discharge
Rechargeable Lithium cells suffer from under-voltage as well as over-voltage. Allowing the cell voltage to fall below about 2 Volts by over-discharging or storage for extended periods results in progressive breakdown of the electrode materials.
Anodes
The copper current collector in the anode slowly dissolves into the electrolyte, increasing the self-discharge rate. When the voltage is increased above 2 v later, these ions may not all reach the current collector. Rather they deposit as copper metal plating on the anode surface. In a manner similar to plating in overcharge, dendrites can develop and cause a short circuit.
Cathodes
Cathodes can contain Lithium Cobalt Oxide, Lithium Manganese Oxide, and Lithium Iron Phosphate. With voltage cycling below and above 2 v, the cathode structure breaks down gradually, these compounds releasing oxygen. This again results in permanent capacity loss.
3. Temperature Effects
Operating at higher and lower than desired temperatures both have adverse effects:
- Low Temperature Operation
Low temperature invariably impedes chemical reaction. This causes an increased source resistance, plating of the anode with lithium metal.
- High Temperature Operation
Accelerated chemical action produces higher currents results in overheating and a breakdown of the solid electrolyte interphase (SEI) layer. This can lead to thermal runaway and battery destruction.
4. Physical Damage
Another way you can damage your battery is outright physical damage; hitting hard, throwing, puncturing, etc., are easy ways of damaging the batteries.
