Materials for Lithium-Ion Battery safety
During the selection of materials for (lithium-ion) batteries, the performance has to be weighed against safety. Markets demand higher performance in terms of capacity, higher current rates, operating life, wide temperature range, charge density, etc. But when it is a question of safety, everything else becomes secondary, hence intense and continuous research into materials.
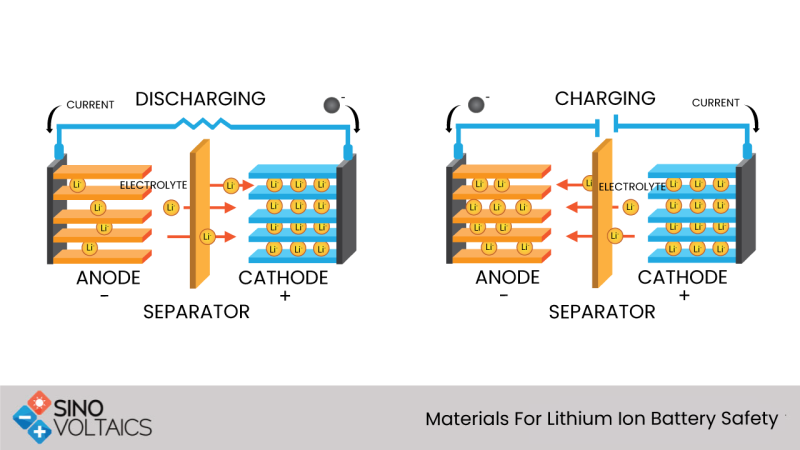
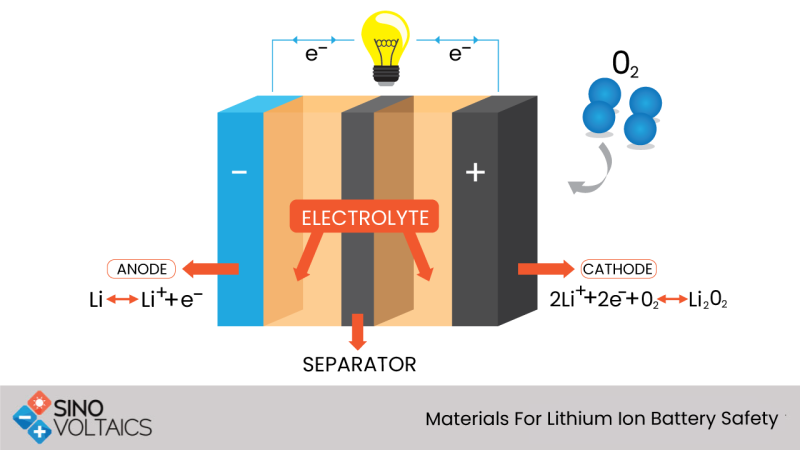
The lithium battery consists of the following parts, which we will discuss in sequence:
- Cathode
- Anode
- Separators
- Electrolyte
1. Cathode Materials
Most of the commercially available LI batteries possess cathodes made from transition metal oxides, e.g., LiCoO2. LCO2 (LCO) and has been in use since 1991 when Sony Corporation introduced its Lithium-ion batteries. Most batteries still use LiCoO2 as cathode material because of its high specific capacity of 155 mAh/g.
However, Cobalt is very expensive, thermally unstable, and toxic. Other materials are LiMn2 O4 (abbreviated LMO), Li(Nix Coy Alz )O2 (abbreviated NCA) and Li(Nix Coy Mnz )O2 (abbreviated NCM).
- NCM has higher specific energy in the range 140-180 mAh/g and high upper voltage cutoff, and it is also more affordable.
- The NCM materials can decompose and release oxygen at high temperatures.
- LMO has a spinel-like structure with better thermal stability and high voltage but suffers low specific capacity (100-120 mAh/g).
- Li FePO 4 (LFP) cathode material has a stable structure, is safer than NCM and LCO, and has a specific capacity of 160 mAh/g with a low charge/discharge rates.
- In research-phase, there are materials with high capacity and high voltage, including Li[Li 1/9 Ni1/3Mn5/9]O2 275 with a specific capacity of 255 mAh/g, carbon-coated LiFePO4 nanospheres and vanadium pentoxide. An increasing percentage of Ni and Li can raise the particular capacity of the cathode material but adversely affects thermal instability.
2. Anode materials
The anode is made either from graphite or Li4 Ti5 O12 (LTO). The theoretical capacity for the graphite is 372 mAh/g and for Li4 Ti5 O12 is 175 mAh/g. The voltage with LTO anode is decreased for a given cathode material, as well as the energy density. However, the LTO anode is more stable with temperature and has a better cycle. The use of LTO anodes are preferred when the energy density is of lesser importance, and graphite remains the popular choice for most batteries where energy density and compactness is desirable. New anode materials with high capacity and high voltage are being researched including silicon, transition metal oxides, silicon oxide, etc. with capacities ranging to 1200 mAh/g. The current drawbacks of these materials are their massive volume expansion (at charge /discharge), dendrite formation, low conductivity, and instability.
References:
- “A review of lithium-ion battery failure mechanisms and fire prevention strategies”, Qingsong Wang et al., Progress in Energy and Combustion Science 73 (2019) 95–131.
- “A failure modes, mechanisms, and effects analysis (FMMEA) of
- lithium-ion batteries”, Hendricks and others, Journal of Power Sources 297 (2015) 113--120