Electrolysis
How to Store? Unfortunately, power cannot be stored, but, we can convert power to energy for regeneration of power later. The most stable form of energy is fuels, including fossil fuels. But we want green fuels- fossil fuels are polluting our environment and causing global warming. The best green fuel is hydrogen. Burning hydrogen produces nothing but water. Spare solar energy can be used to convert water-another abundant resource- to hydrogen (and oxygen) using electrolysis. Stored hydrogen can later be used in fuel cells to generate power. What is Electrolysis? Electrolysis is the splitting of a chemical into its radicals with application of electric current. The substance which breaks up is called the electrolyte. Different chemical electrolytes need different voltages to break them into their radicals. The voltage must be sufficient to overcome the affinity between the two radicals, and then to drive sufficient current. Electrolysis of Water Water, on electrolysis, splits into Hydrogen and oxygen gases. However, pure water conducts current poorly. Unless a very large potential is applied electrolysis of pure water proceeds very slowly limited by the overall conductivity. A larger potential means more heating for the same rate of electrolysis. See more here : http://www1.lsbu.ac.uk/water/electrolysis.html 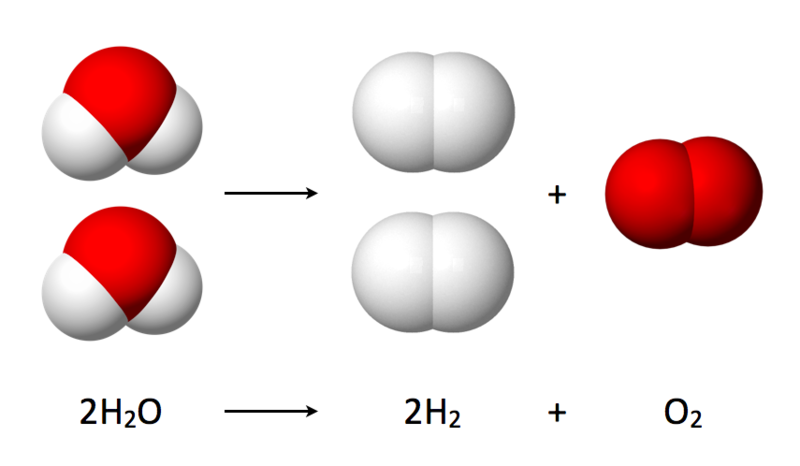 Accelerating Electrolysis of Water Search is on for finding cheap means of fast electrolysis. Various techniques are possible. • Adding an Alkaline Electrolyte- A water soluble chemical is added which readily dissociates into ions and provides conductivity. However, care must be taken in choosing the electrolyte because ions from the electrolyte may end up at the electrodes rather than hydrogen and oxygen. Alkaline solutions of potassium hydroxide or sodium hydroxide are used as electrolytes. A diaphragm separating the electrodes separates product gases while allowing the hydroxide ions from one electrode to the other.
Accelerating Electrolysis of Water Search is on for finding cheap means of fast electrolysis. Various techniques are possible. • Adding an Alkaline Electrolyte- A water soluble chemical is added which readily dissociates into ions and provides conductivity. However, care must be taken in choosing the electrolyte because ions from the electrolyte may end up at the electrodes rather than hydrogen and oxygen. Alkaline solutions of potassium hydroxide or sodium hydroxide are used as electrolytes. A diaphragm separating the electrodes separates product gases while allowing the hydroxide ions from one electrode to the other. 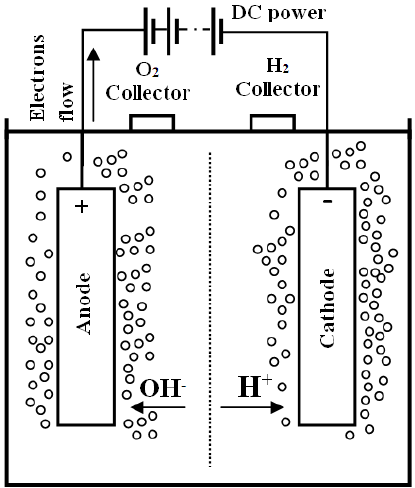 • Photoelectrochemical Water Splitting (PEC) - Sunlight is used directly to split water. To provide the higher voltage required multijunction cells are used. Multijunction cells also convert light more efficiently because they utilize a wider band of solar spectrum. • High Temperature Splitting- High-temperature electrolysis (HTE), or steam electrolysis is a is under investigation. It may prove superior to room-temperature electrolysis because of two reasons: o Heat is supplied instead of electricity. Heat is cheaper. It can be provided directly from the sun, while electricity involves conversion. o The electrolysis is more efficient because of the higher temperatures used. • Polymer electrolyte membrane (PEM) electrolysis is carried out in a cell with a solid polymer electrolyte (SPE) that allows conduction of protons (hydrogen ions), separates the product gases, and provides electrical insulation between the electrodes. The PEM electrolyzer was invented as an improvement on the alkaline electrolyzer. However, recently it has been shown to be even superior. • Catalysts- Various catalysts based on costly metals like iridium and platinum can improve the electrolysis rate. All are costly. However, recently, inexpensive catalysts based on nickel and iron have been discovered which show promise.
• Photoelectrochemical Water Splitting (PEC) - Sunlight is used directly to split water. To provide the higher voltage required multijunction cells are used. Multijunction cells also convert light more efficiently because they utilize a wider band of solar spectrum. • High Temperature Splitting- High-temperature electrolysis (HTE), or steam electrolysis is a is under investigation. It may prove superior to room-temperature electrolysis because of two reasons: o Heat is supplied instead of electricity. Heat is cheaper. It can be provided directly from the sun, while electricity involves conversion. o The electrolysis is more efficient because of the higher temperatures used. • Polymer electrolyte membrane (PEM) electrolysis is carried out in a cell with a solid polymer electrolyte (SPE) that allows conduction of protons (hydrogen ions), separates the product gases, and provides electrical insulation between the electrodes. The PEM electrolyzer was invented as an improvement on the alkaline electrolyzer. However, recently it has been shown to be even superior. • Catalysts- Various catalysts based on costly metals like iridium and platinum can improve the electrolysis rate. All are costly. However, recently, inexpensive catalysts based on nickel and iron have been discovered which show promise.
